Samples’ and their intermediate products’ ID tracking is an important function of LIMS system. Without strict ID naming and following-up rules, it can not only bring confusions to lab operation, but also sometimes, creates many loose ends which can interrupt lab’s working process. Therefore, labs need to be very careful in defining ID naming rules and understand how the IDs are tracked in the LIMS.
After samples are received at the lab, they should be assigned with unique internal IDs, which are different from sample’s external or customer IDs. Bio-LIMS system is flexible in allowing customers to determine their own naming rule for the internal ID. The ID may be associated with the date on which samples are received. Or it can be related to the sequential number for the sample the lab has received. In addition, the lab can use a unique code (for example, one or two characters) in the sample ID to signify sample’s category. For example, the lab can use “C12345” to mark a clinical sample, and use “R23456” to specify a research samples.
In NGS lab, samples need to go through a series of lab operations before being loaded to sequencer. Ideally, it is a one way direction along the lab’s operational process. However, for the samples failed at certain processing steps (for example, failed to pass the QC criteria), they needs to be reprocessed to generate qualified products. If the lab only uses sample’s internal ID in tracking its intermediate products, it creates the problem of having the same ID for failed product and successful product. Some people may think it is not a serious issue, because the lab can just throw away failed one and continue to use the ID for the successful one. However, clinical labs are obligated to record all of the information for future auditing. Simply replacing QC value on the same sample ID will need to make some explanation. In addition, samples failed at certain lab processing step do also carry important information. Having a root cause analysis on the problem will help the lab to make improvement on its operation. Therefore, they should not be simply thrown away.
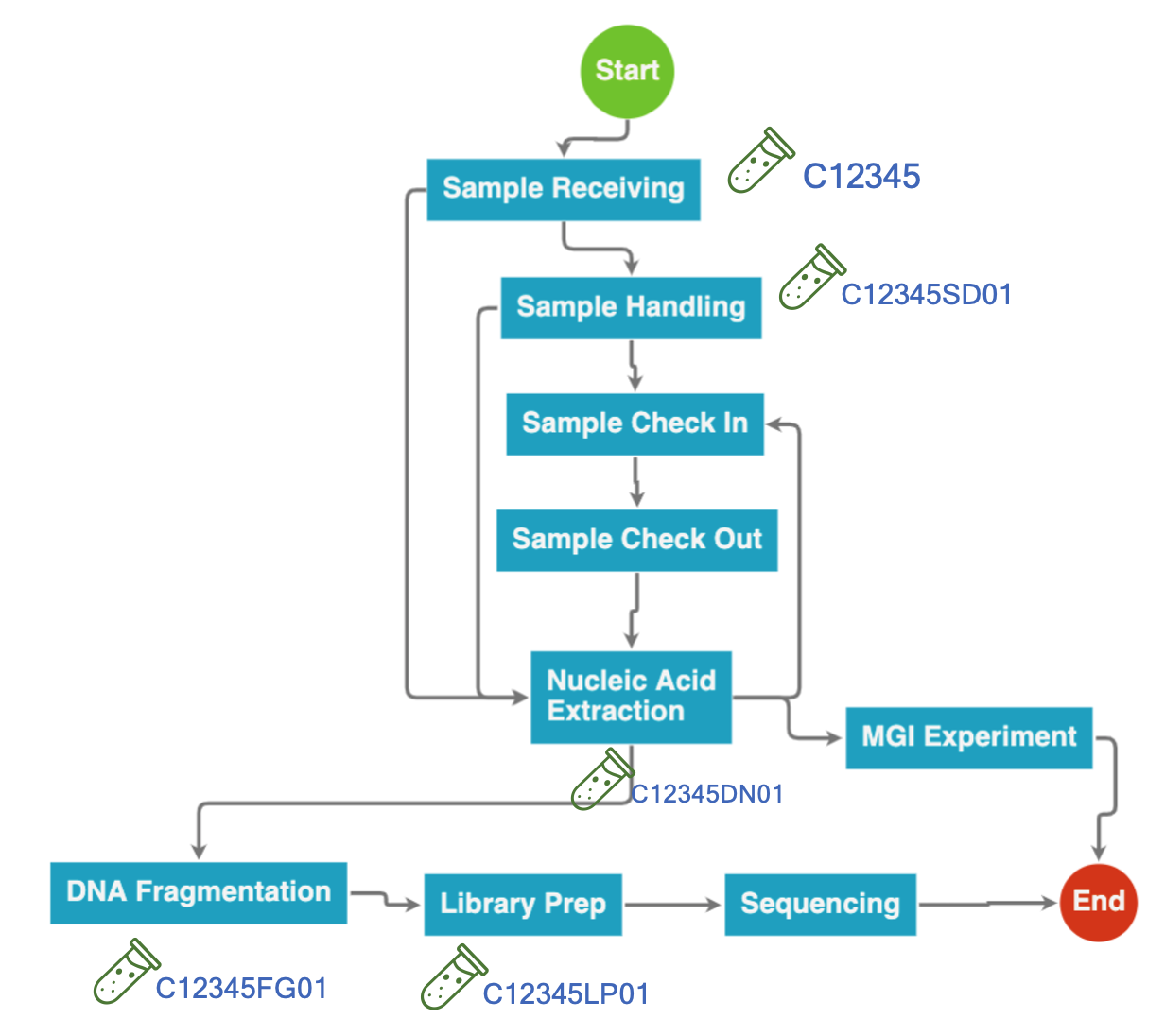
The solution Bio-LIMS has implemented is to uniquely track the intermediate product, whose ID not only carries the source sample ID, but also has the information about the product sample type and a processing series number. For example, for a blood sample, whose internal sample ID is “C12345”, the extracted DNA product will have the ID of “C12345DN01”. If it fails QC and the lab needs to re-extract DNA from the same sample, the product of the second effort will get a new ID of “C12345DN02”. By uniquely tracking intermediate products, Bio-LIMS allows the lab to manage failed samples and avoid confusion in experimental process re-work. For abnormal sample management on Bio-LIMS, please refer to this blog article.