In Next Generation Sequencing (NGS) lab, samples need to go through a series processing steps and quality control (QC) steps before being loaded to flowcell and processed by sequencer. At each processing step, some samples may fail because of certain reason and cannot pass predefined QC criteria. Further operation on the failed samples may affect lab operation and therefore should be handled carefully on LIMS implementation. Here, we present the solution provided by Bio-LIMS and describe different scenarios labs can apply to keep operation smooth.
On Bio-LIMS, lab processing steps are modeled into system modules, each of which is executed by one lab group. For example, Sample Registration module is used by the sample receiving group to register new samples received by the lab. Library Prep module is used by the Library Preparation group, or the authorized lab members, to prepare DNA or RNA libraries. Within each module, samples are processed in batch and perform QC quantification in batch. Successful samples will be marked as “Qualified” and failed samples are marked as “Unqualified” on Bio-LIMS’ user interface.
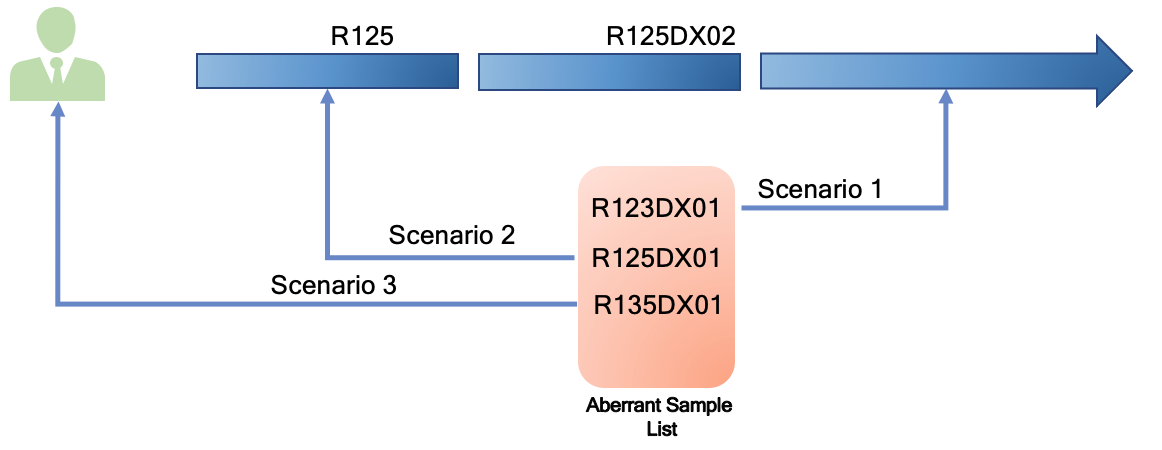
When the experiment is done, Qualified samples will be moved to the downstream modules (according to the predefined lab processing workflow), while Unqualified samples are moved to the module’s Aberrant Sample List, which is individually owned by all of the system modules. By putting Unqualified samples aside, labs can continue to process Qualified samples in the same operation batch downstream without waiting.
Then the question is how to handle the Unqualified samples in the Aberrant Sample list within one module. They are lab specific, but Bio-LIMS has implemented the functions handling the following 3 scenarios, which can cover all of the possibilities:
Scenario 1
Lab operator can double check and decide the Unqualified sample is indeed good and fitful for downstream operation. He/She can then add notes and manually move the sample to downstream step. The corresponding change record is recorded in the system audit log. However, the revived samples may not be able to catch up other samples of the same batch in the downstream operation step and need to operate in a new batch.
Scenario 2
The Unqualified samples (meaning the intermediate product) need to be terminated and lab needs to re-run the experiment to produce another one. If the lab groups still has leftover of the input product, they can use it to re-run the experiment in a new batch. If not, they need to communicate with upstream groups or bio-bank group to ask for additional samples, which may need to go through some of the upstream lab operations. In either case, lab operator can record notes and mark the Unqualified sample “Handled” on Bio-LIMS. Because Bio-LIMS uniquely tracks samples’ and their intermediate products’ IDs, the new sample IDs will not be mixed up with the Unqualified sample IDs.
Scenario 3
The lab may find it does not have enough samples to re-run experiment and therefore, may need to communicate with patient to recollect sample. In this situation, the lab operator will escalate the issue to the Project Management team and mark the Unqualified sample “Handled” on Bio-LIMS.
Abnormal samples should be handled carefully in the lab. By moving Unqualified samples aside and handling them separately, Bio-LIMS allows lab to operate in high throughput manner. Also, with Bio-LIMS’ capability of recording all of the information in its database, labs can pull statistic data and generate managerial reports easily. It will allow lab to find problem earlier and continue to make improvement in its operation.
Pingback: Sample and Intermediate Product ID - Bio-LIMS' Solution - SciTechLink LLC